WASHINGTON — U.S. health officials on Friday endorsed the first blood test that can help diagnose Alzheimer’s and identify patients who may benefit from drugs that can modestly slow the memory-destroying disease.
Related Articles
Minnesota seeks to replace retiring ag mental health counselor
Gene editing helped a desperately ill baby thrive. Scientists say it could someday treat millions
A boy likely died from drinking too much olive brine. A Colorado county tried to make the suspicious case disappear
DeSantis signs a bill making Florida the 2nd state to ban fluoride from its water system
FACT FOCUS: Trump blames other countries for high US drug prices. Experts say it’s not their fault
The test can aid doctors in determining whether a patient’s memory problems are due to Alzheimer’s or a number of other medical conditions that can cause cognitive difficulties. The Food and Drug Administration cleared it for patients 55 and older who are showing early signs of the disease.
More than 6 million people in the United States and millions more around the world have Alzheimer’s, the most common form of dementia.
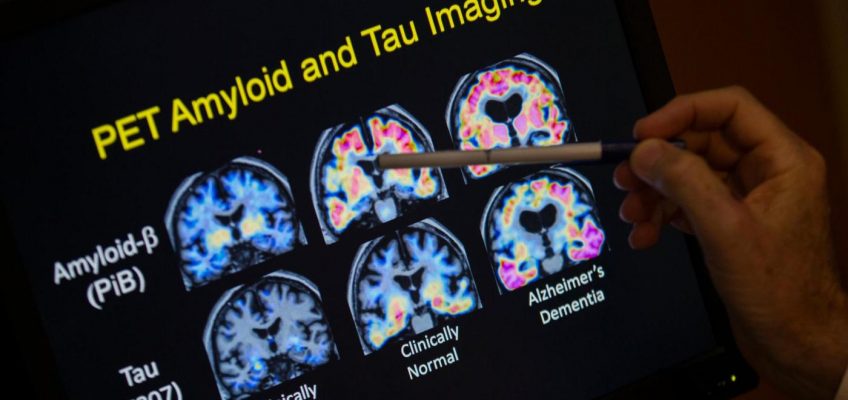
The new test, from Fujirebio Diagnostics, Inc., identifies a sticky brain plaque, known as beta-amyloid, that is a key marker for Alzheimer’s. Previously, the only FDA-approved methods for detecting amyloid were invasive tests of spinal fluid or expensive PET scans.
The lower costs and convenience of a blood test could also help expand use of two new drugs, Leqembi and Kisunla, which have been shown to slightly slow the progression of Alzheimer’s by clearing amyloid from the brain. Doctors are required to test patients for the plaque before prescribing the drugs, which require regular IV infusions.
“Today’s clearance is an important step for Alzheimer’s disease diagnosis, making it easier and potentially more accessible for U.S. patients earlier in the disease,” said Dr. Michelle Tarver, of FDA’s center for devices.
A number of specialty hospitals and laboratories have already developed their own in-house tests for amyloid in recent years. But those tests aren’t reviewed by the FDA and generally aren’t covered by insurance. Doctors have also had little data to judge which tests are reliable and accurate, leading to an unregulated marketplace that some have called a “wild west.”
Several larger diagnostic and drug companies are also developing their own tests for FDA approval, including Roche, Eli Lilly and C2N Diagnostics.
The tests can only be ordered by a doctor and aren’t intended for people who don’t yet have any symptoms.
AP Medical Writer Lauran Neergaard contributed to this story
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.

Leave a Reply